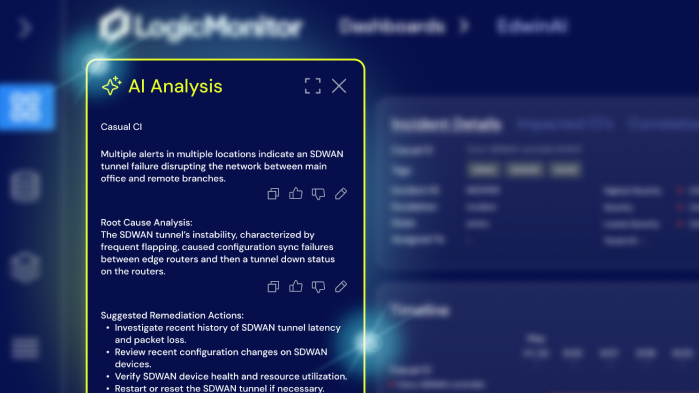
Hybrid Observability for Life Sciences
Life Sciences IT Monitoring: Secure, Scalable, AI-Powered
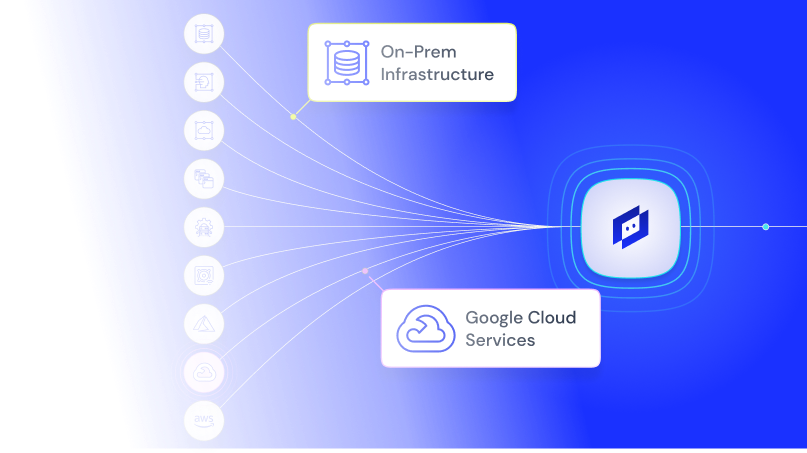
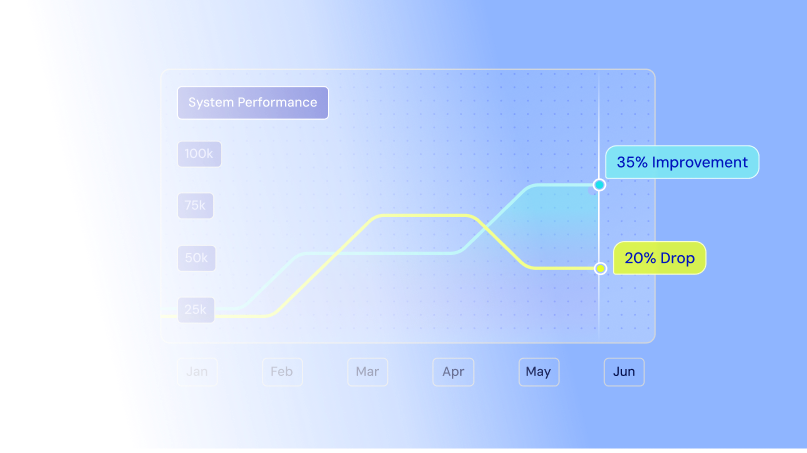
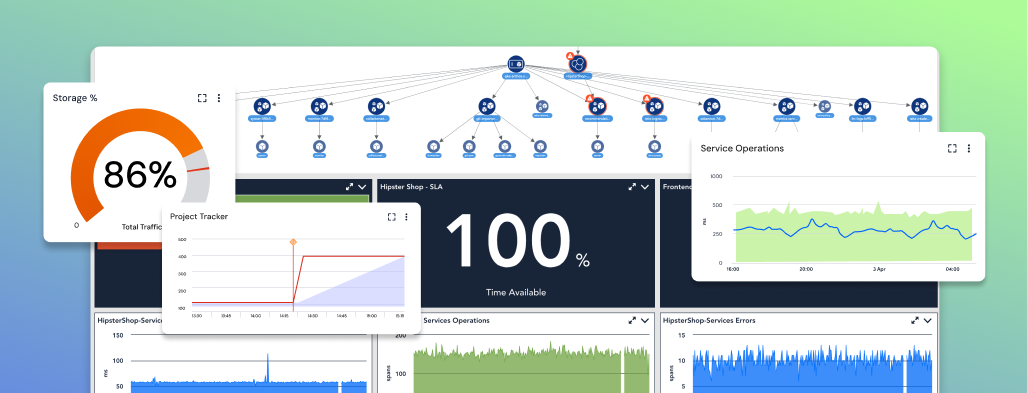
Keep research on track and systems secure across cloud, on-prem, and edge environments with real-time insights, AI-driven alerting, and unified visibility.